Uranium from Oceans: A Sustainable Energy Revolution
Revolutionizing Uranium Extraction: A Game-Changer for Nuclear Energy

Recent breakthrough research, featured in the journal ACS Central Science, unveils an innovative material capable of revolutionizing the extraction of uranium ions from seawater through electrochemical processes. This groundbreaking development promises to significantly enhance the efficiency of extracting elusive uranium ions, surpassing conventional methods by a considerable margin.
The Vast Reservoir of Seawater Uranium
As per the Nuclear Energy Agency's findings, our planet's oceans hold an astounding 4.5 billion tons of uranium in the form of dissolved uranyl ions. This underwater uranium reserve dwarfs the terrestrial deposits by more than a thousandfold. However, tapping into this vast resource has proven to be an arduous challenge, primarily due to the limitations of existing materials, which lack the necessary surface area to effectively capture these ions.
The Ingenious Solution
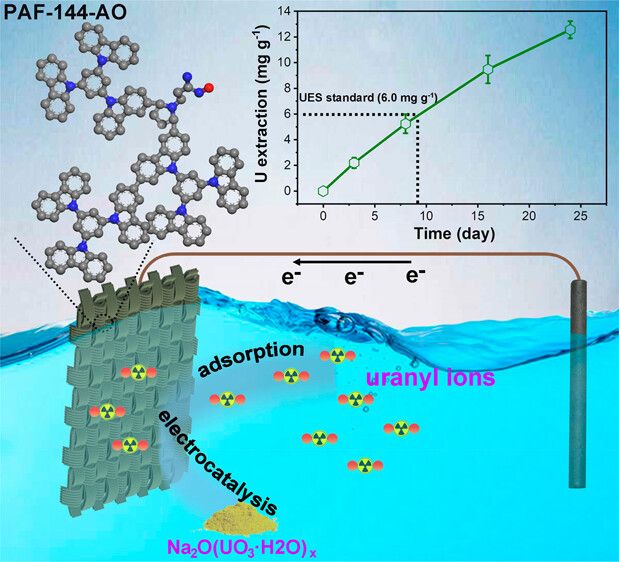
Enter the ingenious invention crafted by Rui Zhao, Guangshan Zhu, and their team from Northeast Normal University in Changchun. They have devised an electrode material endowed with a multitude of microscopic crevices and recesses, perfectly suited for the electrochemical entrapment of uranium ions from seawater.
The production of these extraordinary electrodes commences with the weaving of a flexible cloth using carbon fibers. This cloth is then coated with two specialized monomers, which undergo polymerization. Subsequently, the cloth is treated with hydroxylamine hydrochloride, adding amidoxime groups to the polymers. The inherent porous structure of the cloth provides numerous minuscule pockets for the amidoxime groups to nestle within, facilitating the efficient trapping of uranyl ions.
Transformative Experimentation
In rigorous experiments, the researchers deployed the coated cloth as a cathode, submerging it in both naturally sourced seawater and seawater spiked with uranium. They introduced a graphite anode and initiated a cyclic current between the electrodes. Over time, conspicuous yellow uranium-based precipitates began to accumulate on the cathode cloth.
Remarkably, in tests involving seawater collected from the Bohai Sea, these innovative electrodes successfully extracted an impressive 12.6 milligrams of uranium per gram of coated, active material over the course of 24 days. This capacity surpassed the performance of most other materials tested for uranium extraction by the research team.
Moreover, the use of electrochemistry to capture these ions proved to be approximately three times faster than the natural accumulation method on traditional cloths.
A Game-Changing Approach
In the perspective of the researchers, this groundbreaking work introduces an exceptionally effective means of capturing uranium from seawater. It holds the potential to open up the world's oceans as a new source of nuclear fuel supply, marking a significant milestone in the quest for sustainable energy solutions.
This remarkable achievement not only addresses the challenges of uranium extraction but also contributes to the advancement of clean and renewable energy. It serves as a testament to human ingenuity and our commitment to harnessing the vast resources that our planet offers.
Conclusion
The discovery of this novel material, designed for the efficient extraction of uranium from seawater, represents a remarkable step forward in the field of nuclear energy. With its microscopic nooks and crannies, this innovative electrode material promises to make uranium extraction from the oceans a viable and sustainable option for meeting the world's growing energy demands. As we continue to explore alternative energy sources, the ocean's potential as a uranium reservoir is now more accessible than ever before.